Resources
Next generation sequencing indeed created a wide universe of data and opportunities in clinical diagnostics. To help you navigate this universe with confidence, to exploit the full potential of the different applications and to enable a smooth take-off with our varvis® software, our team summarized some basic knowledge, expert tips and tricks, and latest insights for your convenience. We are here to support your onboarding with all the training you need.
Workshops and presentations on demand
Listen to our expert talks at the latest conference workshops and presentations.
Watch our varvis® webinars on demand to learn about latest developments, best practices and deep-dive sessions.
varvis® academy
Onboarding, training and education - right at your fingertips.
varvis® blog

Update to gnomAD v4.1: Key features
by Dr. Roberta Trunzo, August 27, 2024
The varvis® software now includes the latest version of the Genome Aggregation Database (gnomAD), providing updated annotations for whole exome and whole genome data. This article describes the enhancements and implications of the update to the new version 4.1, particularly for clinical diagnostics.

The varvis® Software: The first genomics end-to-end software certified as IVDR Class C device
by Dr. Ben Liesfeld, May 31, 2024
Genetic diagnostic laboratories now have access to the first complete genomics software solution which is certified as a Class C device under IVDR. This will significantly reduce the effort required for legally compliant documentation of in-house tests.
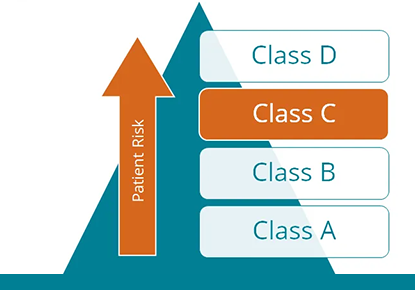
Q&A: The role of the IVDR conformity assessment in genetic diagnostics
by Dr. Ben Liesfeld & Dr. Sonja Strunz,
May 31, 2024
Now that the regulation on in-vitro diagnostic devices (IVDR) explicitly regulates in-house devices, or laboratory-developed tests (LDTs), in the European Union, the selection of properly CE-labeled devices is increasingly important to health institutions like genetic testing laboratories.'